Background
Despite being the only curative option for many hematologic malignancies, allogeneic hematopoietic cell transplant (HCT) has been plagued by health disparities due to differences in access, donor availability, and outcomes across racial and ethnic minority populations (REM) (Gragert, NEJM, 2014). Even when a matched donor is available, HCT outcomes for REM patients are historically worse compared to non-Hispanic white (NHW) patients. (Landry, Stem Cell Investigation, 2021). The development of post-transplant cyclophosphamide (PTCy) for graft-versus-host disease (GVHD) prophylaxis has led to increased use of HLA haploidentical (haplo) or mismatched unrelated donors (MMUD) with comparable outcomes to HLA matched HCT (McCurdy, Advances in Hematology, 2015). While PTCy allows for almost every transplant-eligible REM patient to have a suitable donor, there is limited data evaluating whether this transplant platform results in similar outcomes for REM patients compared to NHW patients. The aim of this study was to investigate if disparities in transplant outcomes still exist between REM patients and NHW patients who received an HLA mismatched transplant with PTCy.
Methods
We retrospectively analyzed 302 consecutive adult patients who underwent haplo or MMUD HCT with PTCy at Moffitt Cancer Center between 2014 and 2022. All endpoints were compared between four groups: NHW, Hispanic-White, Black, and Other (Asian, biracial, or multiracial.)
Results
In this cohort, 59% (n=178) of patients identified as NHW, 14% (n=41) as Hispanic-White, 16% (n=48) as Black, 7% (n=21) as biracial or multiracial, and 4% (n=11) identified as Asian. Most patients were male (57%). The median age of all patents was 58. The most common indication for transplant was acute myeloid leukemia (48%), myeloablative conditioning (MAC) was utilized in 42%, and 89% received peripheral blood stem cells grafts. REM patients were more likely to be <65 years of age (60% vs 66-90%, p<0.001), female (36% vs 50-54%, p=0.04), and receive MAC conditioning (33% vs 40-71%, p<0.001). Median follow-up was 21 months.
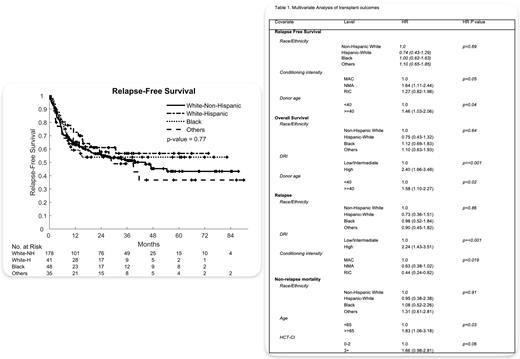
In all clinical outcomes, no significant differences were observed between NHW and REM patients. At day 100, the cumulative incidence of Grade II-IV aGVHD was 37% [95% CI 30-44%] for NHW patients, 32% [95% CI, 18-46%] for Hispanic-White patients, 27% [95% CI, 15-40%] for Black patients, and 26% [95% CI, 13-41%] for patients of Other races (p= 0.38). The cumulative incidence of moderate to severe cGVHD at 2 years was 21% [95% CI 15-28%] for NHW patients, 25% [95% CI, 13-39%] for Hispanic-White, 17% [95% CI, 7.8-29%] for Black, and 27% [95% CI, 13-42%] for patients of Other races (p = 0.80). At 2 years, the probability of relapse was 25% [95% CI 19-32%] for NHW patients, 24% [95% CI, 12-39%] for Hispanic-White patients, 27% [95% CI, 15-40%] for Black patients, and 18% [95% CI, 7.1-33%] for patients of Other races (p = 0.97). During the same time interval, non-relapse mortality (NRM) for NHW patients, Hispanic-White patients, Black patients, and patients of Other races were 19% [95% CI, 13-25%], 15% [95% CI, 5.9-27%], 19% [95% CI, 9.4-32%], and 24% [95% CI, 11-40%] (p = 0.73), respectively. At 2 years, relapse free survival (RFS) was 56% [95% CI 49-64%] for NHW patients, 61% [95% CI, 47-79%] for Hispanic-White patients, 54% [95% CI, 41-71%] for Black patients, and 58% [95% CI, 43-78%] for patients of Other races (p = 0.77). Comparably, OS at 2 years for NHW, Hispanic-White patients, Black patients, and patients of Other races was 64% [95% CI, 57-72%], 72% [95% CI, 60-88%], 53% [95% CI, 40-71%], and 63% [95% CI, 49-83%] respectively after two years (p = 0.92).
In multivariate analysis, race/ethnicity had no significant impact on incidence of Grade II-IV aGVHD (p= 0.40), moderate/severe cGVHD (p = 0.80), rates of relapse (p = 0.86), NRM (p= 0.77), RFS (p = 0.69), or OS (p = 0.64) (Table).
Conclusions
These results show that the use of HLA mismatched donor HCT with PTCy achieves comparable clinical outcomes between REM and NHW patients. Our study suggests that PTCy effectively contributes to equitable healthcare in the setting of allogeneic HCT for REM patients.
Disclosures
Faramand:Kite: Research Funding; Gilead: Research Funding. Lazaryan:Sanofi: Consultancy, Other: Consultancy/speaker and scientific advisory board. Liu:BioLineRx: Membership on an entity's Board of Directors or advisory committees. Jain:Myeloid Therapeutics: Consultancy, Honoraria; Loxo@Lilly: Research Funding; Incyte: Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding. Nishihori:Medexus: Speakers Bureau; Moffitt Cancer Center: Other: Personal fees from Karyopharm and Novartis outside the submitted work. Bejanyan:Orca Bio: Consultancy, Research Funding; CareDx Pharma: Consultancy, Research Funding; CRISPR: Consultancy, Research Funding; Sanofi: Consultancy, Research Funding; CTI BioPharma: Consultancy, Research Funding; Medexus Pharmaceuticals: Consultancy, Research Funding; Magenta: Consultancy, Research Funding. Elmariah:Bristol Myers Squibb: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal